Результаты применения VIDATOX® 30 CH при лечении онкологии.
Использование Vidatox® 30 CH
Результаты использования Vidatox® 30 CH больными с онкологическими патологиями.
Автор: Магистр. Фабио де Хесус Линарес Pazos.
LABIOFAM Сьенфуэгос.
Введение:
Рак является одной из основных причин смертности во всем мире: в 2005 году 7,6 млн. летальных исходов были вызваны раком, и предполагается, что более 84 миллионов умрут к 2015 году, хотя более 40% всех случаев рака являются прогнозируемыми.
Эта патология была в течение более чем трех десятилетий второй причиной смертности на Кубе для всех возрастных категорий, включая детей с первых лет жизни, при этом ситуация изменилась немного в последнее время.
Различные исследователи изучают патологию рака и зависимоть от общей смертности и / или риска умереть от болезней. Все они подтвердили устойчивый рост заболеаемости онкологией в настоящее время.
В целях своевременного выявления болезни, лечение и ухода за пациентами, страдающими от рака, Международный центр исследований рака (МАИР) ВОЗ обеспечивает ученых не только научными данными о причинах и механизмах развития болезни, но и способствует исследованиям, чтобы найти способ ее лечения.
В этой связи, Куба представила препарат для применения альтернативных методов лечения онкологии - как яд скорпиона Rhopalurus junceus, который является в гомеопатическим средством.
Использование яда эндемичных видов скорпиона восходит к началу девятнадцатого века, когда яд был впервые опробован в качестве потенциального противоопухолевого средства. В этой связи, исследования, проведенного Business Group LABIOFAM доказали, что яд scorpion Rhopalurus junceus и фракции молекулярной массы ниже 5 кДа оказывает значительное подавляющее воздействие на опухолевые клетки эпителиального происхождения. Это делает Vidatox® 30 СН дополнительным средством для лечения рака.
Цель настоящего документа является предоставление научных доказательств, что характеризуют влияние Vidatox® 30 CH для конкретного онкологического лечения.
Методы
Ретроспективное исследование о влиянии Vidatox® 30 CH проводилось на 174 добровольных пациентов на Кубе, которые проходили лечение препаратом с 2008 года до 2010 года при дозе 5 капель каждые 12 часов под язык.
Критерии
1. Пациенты были диагностированы с помощью различных средств и методик.
2. Пациенты проходили различные виды онкологических лечений.
3. Пациенты проходили предварительное анкетирование.
Исключения:
1. Проводились исследования на пациентах, у которых был подтвержден онкологический диагноз.
2. Все пациенты имели отрицательный тест на беременность.
3. Исключались дети в возрасте до одного года
АНГЛИЙСКАЯ (ПОЛНАЯ) версия:
Use of VIDATOX® 30 CH
Results of the use of VIDATOX® 30 CH in patients carrying oncological pathologies.
Autor: MSc. Fabio de Jesús Linares Pazos.
LABIOFAM branch Cienfuegos.
Introduction:
Cancer is one the main causes of mortality worldwide: in 2005 7,6 million deaths caused by cancer were reported, and it is estimated that additional 84 millions will die by 2015, even though more than 40% of all cancer are predictable1. This pathology has been, for more than three decades, the second cause of death in Cuba for all ages and the first regarding lost years of life, though the situation has changed a little bit.2
Different researchers have studied this important pathology and the cancer caused general mortality and/or risk of dying from this disease in the Cuban population3-5. All of them confirmed the sustained increased of this disease to the present date.
In order to improve timely detection, treatment and care of patients suffering from cancer, the International Center of Cancer Research (CIIC) of WHO provides not only scientific evidence on the causes and mechanisms of the development of the disease, but also favors researches to find a way to treat it. In this regard, Cuba has made incursions in the application of alternative therapies, like the venom of scorpion Rhopalurus junceus, in homeopathic presentation.
The use of the venom of this endemic species dates back to the beginning of the XIX century, when was seen for the first time the potential as antitumoral agent. In this regard, researches conducted by the Business Group LABIOFAM proved that, both the venom of scorpionRhopalurus junceus and the fractions of molecular mass lower than 5 KDa, have a significant toxicity on tumoral cells of epithelial origin. This, coupled with the good used of homeopathic therapies to favour the self-healing reaction of a sick organism, make VIDATOX® 30 CH a complementary option to considered for the treatment of cancer.
Having this in mind, the objective of this paper is to provide scientific evidence that contributes to characterize the effect of VIDATOX® 30 CH as coadjutant of the specific oncological therapy.
Methods
A retrospective study on the effect of VIDATOX® 30 CH was conducted on 174 volunteer patients that received the treatment from 2008 until 2010. The dose administered in all cases was of 5 sublingual drops every 12 hours
Inclusion criteria
- To have been diagnosed by one or various diagnosis means.
- To have been submitted to different established oncological treatments.
- To have answer the pre-elaborated questionnaire.
Exclusion criteria
- Not having confirmative diagnosis of malign ant tumoral pathologies.
- To a have positive diagnosis for pregnancy
3. Children under one year of age
Indicators to bear in mind
Demographic characteristics of the population being studied
Results
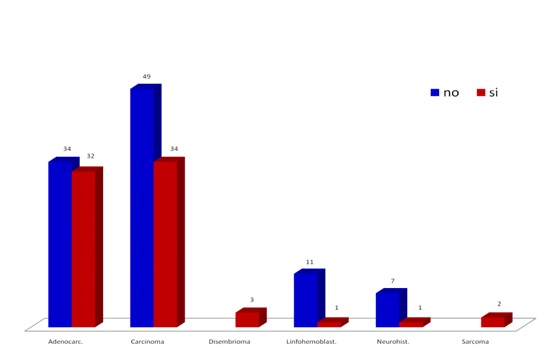
Fig. 1 shows the distribution of the simple by neoplasia family and metastasis reference in each case. Note a higher presence of carcinomas and adenocarcinomas and prevalence of metastasis. This distribution by neoplasia family of the sample matches the RNC5 reports.
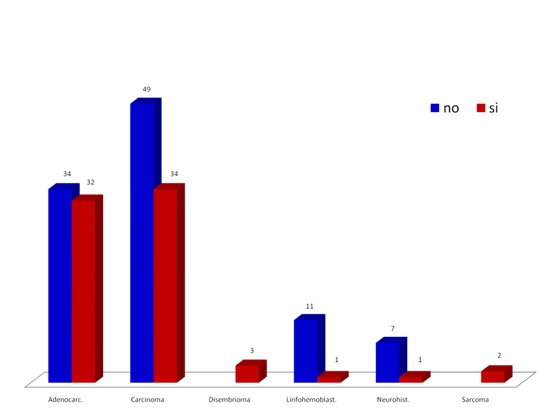
Figure 1. Distribution of neoplasic families and metastasis in the studied population.
The country and sex ration is shown in Figure 2. note that Cuban patients prevail with 128 cases for 73,56% followed by Mexico with 9.20 % and Colombia with 6,90%. Regarding sex there is a slight predominance of women with 92 cases over men with 82, for 1.1 ratios. This ratio differs in Mexico with 12 men and 4 women under treatment for a ration of 3.
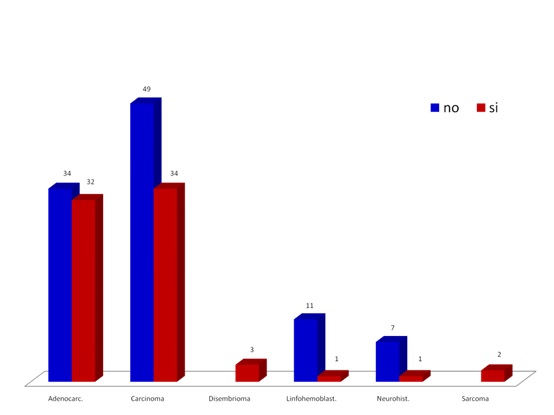
Figure 2. Distribution of patients by country and sex.
Figure 3 shows oncological treatments received by patients object of this study who are grouped by neoplasic families, the treatments include surgery, chemotherapy, radiotherapy, therapeutic vaccines. Note the predominance of chemotherapy, surgery and radiotherapy, similar to international situation.1 The cases of adenocarcinoma, carcinoma and neurohistone present patients for each therapeutic variant mentioned.
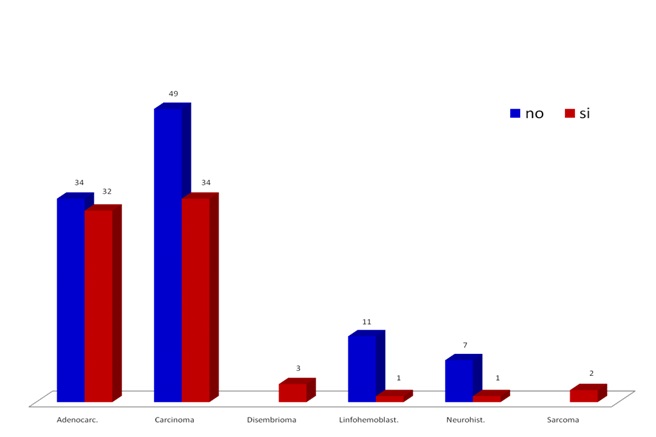
Figure 3 Specific oncological treatments according to the oncological family in the studied sample.
Fig. 4 shows the distribution of patients by primary affected organ and oncological family, with predominance of lung, prostate, colon, breast, lymphoid tissue, pancreas, uterus, brain, skin, recto, stomach and testicle among the 33 indexed locations.
Some of the results obtained through the RNC informative system are:
- The ten most frequent cancer locations in Cuba: Lung, prostate and swing rank the first for men and breast, skin and lung are the first for women.
- Cancer mortality by primary location distribution: Lung cancer, prostate and colon for men and lung, breast and colon for women rank the first.
- Among the main locations we find malignant tumors of trachea, bronchus, and lung (22%) and colon and rector (9%). Prostate (19%) is included for men and breast (15%) and uterus (6%) for women. 1 % of cáncer cases and deaths ocurred in children. The main cancers for children less than 15 years of age are leukaemia, lymphomas and kidney tumors.
The results of our simple match what is established by RNC.
Tables # 5, # 6 and # 7 show the symptoms reported by sex according to the primary organ and the improvements in follow up consultations.
Due to the lack of imageonological reports and other tests of confirmation of the evolution of these patients, the information provided here is presented based on symptoms presented at the beginning and modified or not, by the effect of our drug, which is being studied.
We have not intentionally adapted the symptoms to the classic medical terminology and take them as stated in discharge sheets, summarized medical records and case studies conducted by us, recording a total of 57 different symptoms, which are shown in the above-mentioned tables and grouped by affected primary organ. The organs with higher prevalence are shown in the study.
The most important symptoms for each case are:
- Lung (effectiveness on symptoms 100 %)
- Respiratory failures, thoracic pain, coughing, worsening of symptoms by effort, anorexia, feeling down
- Prostate (effectiveness in symptoms 100%)
- Urinating problems, pain, hematuria, problems to defecate, anorexia, loss of weight
- Colon (effectiveness on symptoms 100 %)
- Pain, constipation, problems to defecate, diarrhea, bleeding, anorexia
- Breast (effectiveness on symptoms 100 %)
- Pain, general discomfort, breeding problems
- Utrerus
- Pain, vaginal flow, inflammation, breeding
- Lymphoid tissue (effectiveness on symptoms 100 %)
- Hematological disorders, anemia, breeding difficulties, difficulties to swallow solid food
- Pancreas (effectiveness on symptoms 100 %)
- Inflammation, anemia, jaundice, increased glycerin
- Brain (effectiveness on symptoms 100 %)
- Weakness in limbs, sight reduction, speaking problems, difficulties to swallow solid food
Note: For all cases we have removed from the list those patients who were and remain asymptomatic
Table 8 shows the survival of sampled patients from the date of diagnosis for each case until February 2010, by age and sex. Note there is a predominance of patients under 60 and more female cases up to 64 and more patients whose survival is more than 25 months.
|
Sex |
Survival in months |
|||||||||
|
Age |
f |
m |
n |
% |
<=6 |
12 |
18 |
24 |
>25 |
n |
|
<60 |
56 |
34 |
90 |
52% |
5 |
11 |
16 |
13 |
46 |
90 |
|
60-64 |
10 |
15 |
25 |
14% |
2 |
4 |
1 |
3 |
12 |
25 |
|
65-69 |
6 |
8 |
14 |
8% |
1 |
3 |
3 |
7 |
14 |
|
|
70-74 |
8 |
12 |
20 |
11% |
4 |
5 |
2 |
1 |
8 |
20 |
|
75-80 |
7 |
5 |
12 |
7% |
2 |
2 |
1 |
8 |
12 |
|
|
>80 |
5 |
8 |
13 |
7% |
3 |
3 |
1 |
2 |
5 |
13 |
|
n |
92 |
82 |
174 |
14 |
26 |
25 |
23 |
86 |
174 |
|
Table 8.
Analysis by groups of population
Adults (25-59 years): This group represents 50% of the population. About 20% of deaths occurred at these ages or a rate of 2.8 per 1 000 inhabitants in 2000. the first causes of death were malignant tumors, heart diseases and accidents with 57% deaths. For all cases the male mortality prevailed except for malignant tumors.
Senior adults (60 years or more): 75% of deaths occurred in this group in 2000. The main causes were heart diseases, malignant tumors and cerebrovascular diseases with 63% of the total.
Table 9 shows the improvement of patients in follow up consultations for 3, 6, 9, 12 months and more than one year. The highest percentages are in the groups under or equal to 60 and from 60 to 64 with more cases of improvement after six months of treatment, followed by improvement at three months. This group includes many of the patients that start the treatment in the last semester of 2009, followed then by the group that keeps improvement up to 9 month.
|
Sex |
Improvement in months |
||||||||
|
Age |
f |
m |
n |
% |
3 |
6 |
9 |
12 |
+ 1 year |
|
<60 |
56 |
34 |
90 |
52% |
17 |
27 |
14 |
7 |
5 |
|
60-64 |
10 |
15 |
25 |
14% |
4 |
10 |
4 |
2 |
2 |
|
65-69 |
6 |
8 |
14 |
8% |
1 |
3 |
4 |
1 |
1 |
|
70-74 |
8 |
12 |
20 |
11% |
4 |
2 |
1 |
1 |
3 |
|
75-80 |
7 |
5 |
12 |
7% |
2 |
5 |
1 |
||
|
>80 |
5 |
8 |
13 |
7% |
2 |
7 |
1 |
1 |
2 |
|
n |
92 |
82 |
174 |
30 |
54 |
25 |
12 |
13 |
|
Table 9.
Table 10 calculates the survival of the sample by the actuarial method, determining an accumulated probability of survival of 96%.
|
time period |
Alive at the beginning of the time period |
Deaths during the time period |
Alive abandonment or loss of follow up |
probability of death or event |
Probability of being free in the event |
Accumulated probability of survival |
|
months |
(n) |
(d) |
(w) |
q = d / (n[w/2]) |
pi = 1 – q |
s = pi · pi-1 |
|
3 |
174 |
1 |
0.01 |
0.99 |
0.99 |
|
|
6 |
84 |
2 |
40 |
0.03 |
0.97 |
0.96 |
|
9 |
25 |
0.00 |
1.00 |
0.96 |
||
|
12 |
12 |
0.00 |
1.00 |
0.96 |
||
|
12 + |
13 |
0.00 |
1.00 |
0.96 |
||
|
taken from follow up consultations |
||||||
Table 10.
Table 11 refers to those patients not suitable for a specific oncological treatment due to the advanced age and prognosis. The table reflects the survival index by grouping them by affected primary organ and evaluating them at scales of under or equal to six months and up to 25 months, with a predominance of patients with lung, colon, liver, pancreas and prostate cancer.
|
|
Survival |
||||||
|
Organ |
<=6 |
12 |
18 |
24 |
>25 |
n |
% |
|
Brain |
1 |
1 |
3% |
||||
|
Colon |
1 |
1 |
1 |
3 |
9% |
||
|
Liver |
1 |
2 |
3 |
9% |
|||
|
Breast |
1 |
1 |
3% |
||||
|
Mediastinum |
1 |
1 |
3% |
||||
|
Nerve. |
1 |
1 |
3% |
||||
|
Pancreas |
1 |
1 |
1 |
3 |
9% |
||
|
Prostate |
1 |
1 |
1 |
3 |
9% |
||
|
Lung |
2 |
2 |
4 |
3 |
2 |
13 |
37% |
|
Recto |
1 |
1 |
3% |
||||
|
Blood |
1 |
1 |
3% |
||||
|
Lymphoid tissue |
1 |
1 |
3% |
||||
|
Thyroid |
1 |
1 |
3% |
||||
|
Vagina |
1 |
1 |
3% |
||||
|
Bladder |
1 |
1 |
3% |
||||
|
n |
6 |
6 |
6 |
8 |
9 |
35 |
|
Table 11.
Table 12 shows the analgesic effect of our medicine in patients referring pain, according to the scale established in the Haematology and Orthopaedics Services of “Hermanos Ameijeiras” Clinical Surgical Hospital. A significant improvement effect is note in patients with severe pain (2 in scale) 62%, taking them to the minimal or moderate pain condition (1 in scale) 62% or no pain (0 in scale) 27%. It also modified the disabling pain (3 in scale) of three of the five patients included in this group of severe pain (2 in scale)
Analgesic effect
|
Scale |
Before |
% |
After |
% |
|
|
0 |
17 |
27% |
|||
|
1 |
19 |
30% |
39 |
62% |
|
|
2 |
39 |
62% |
5 |
8% |
|
|
3 |
5 |
8% |
2 |
3% |
|
|
63 |
63 |
||||
|
Table 12. |
|||||
|
Note: Scale established in the Haematology and Orthopaedics Services of “Hermanos Ameijeiras” Clinical Surgical Hospital |
|||||
Generally, patients who have received the sample have witnessed :
- Pain improvement
- Inflammation improvement
- Improvement of hematological parameters
- Improvement of appetite
- General improvement
- Improvement of function in affected organs and systems
- Weight gaining
- Decreased “coughing.
- Desire if living
Conclusions:
Using this homeopathic biotherapy, we have:
- Improved the quality of life.
- Increase survival.
Without going through the unwanted symptoms caused today by radiation and cytostatic therapies, our patients receive.
Bibliography:
- WHO. World Day against Cancer: action to prevent 8 million cancer deaths from now to 2015. Available in: http://www.who.int/mediacentre/news/
releases/2006/pr06/es/index.html.
- Ministry of Public Health; National Division of Statistics and Medical Records. Health Statistical Annual Report. 1970 – 2006. Havana. (Cuba): MINSAP; 1971 – 2007.
- Mendoza M, Caceres CL, Jimenez H. Cancer mortality in Camagűey, from 1980 to 1984. Rev. Cubana Oncol. 1987; 3 (3).
- Gonzalez L, Lemes JJ. Characteristics of cancer mortality. Granma 2000. Cuban magazine of oncology. 2000; 16 (1): 21 – 3.
- Sanso S. F., Alonso G. P., Torres V. R. Cancer Mortality in Cuba. Cuban Magazine on Public Health. 2010; 36 (1).






.jpg)
.jpg)
.jpg)
.jpg)
